
Investor relations > Company profile

Why wound healing?
Tissue Therapies Limited has identified that there is a significant need in wound care for improving the outcome of hard to heal wounds. Hard to heal wounds are defined as wounds that have not responded to standard therapy in an orderly and timely manner [1]. This type of delayed healing occurs in a variety of wound types (venous leg ulcers, arterial leg ulcers, mixed leg ulcers, diabetic foot ulcers and pressure ulcers) [1,2,3].
Treatment of hard to heal wounds is demanding on both patients and healthcare providers, with significant financial impact on the healthcare systems and patient quality of life. For example, conservative costs to the UK National Health Service (NHS) are estimated at £2-3 billion per year. In the United States, hard to heal wounds effect 6.5 million patients and is estimated to cost in excess of US$25 billion annually [4]. In Germany, according to cautious estimates, approximately 3-4 million people suffer from hard to heal wounds and costs associated are high for the Statutory Health Insurance (SHI) funds, the economy and patients [5]. The average annual cost to the SHI per patient for the treatment of venous leg ulcers alone was estimated at €7630.70 and the annual cost to the patient was estimated at €1027 [5]. In 2007, a UK hospital audit found that the drivers behind such high costs were [6];
- The relatively high incidence of non-healing wounds (1 in 4 wounds had been non-healing for at least six months);
- The prevalence of infections associated with non-healing wounds, resulting in longer hospital related care;
- Nursing time, was associated 33-41% of the total cost of care and the cost of wound dressings were associated with 17-22% of the total cost of care.
Clearly there is an opportunity to provide a technological solution that reduces the time and resources taken to resolve hard to heal wounds, in addition to providing improved patient outcomes.
Products
Tissue Therapies Limited has a worldwide exclusive liscence from the Queensland University of Technology to commercialise VitroGro® ECM. VitroGro® ECM is a new treatment for hard to heal wounds that is used in conjunction with standard care, including moist wound dressing and compression. With VitroGro® ECM, Tissue Therapies Limited is placed to meet the need for reducing the burden of hard to heal wounds for both the patients and the healthcare system.
What is VitroGro® ECM and how does it help?
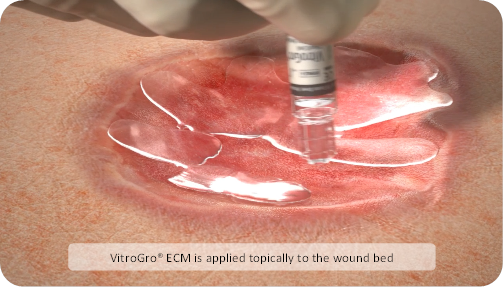
VitroGro® ECM is a topically applied, biomimetic scaffold, comprising a synthetic extracellular matrix (ECM) protein.
How it works:VitroGro® ECM replaces the degraded matrix of a hard to heal wound. VitroGro® ECM binds to a prepared wound bed and provides a physical structure (a scaffold) for cell attachment, which is a primary requirement for subsequent cell functions critical for healing, such as cell proliferation and migration [8].
Optimisation: VitroGro® ECM is an ideal ECM replacement
One of the characteristics of hard to heal wounds is prolonged inflammation, which damages the native ECM that would normally guide the wound healing process [7,8,9,10]. Replacement of this damaged ECM is a beneficial strategy for treating hard to heal wounds [8]. An ideal ECM replacement would be one that closely approximates the structure and function of the ECM it is replacing [8].
VitroGro® ECM is ideal as an ECM replacement because its design is based on proteins that are present in the physical structure of healthy ECM in the early stages of normal wound healing.
VitroGro® ECM: summary of clinical results
In the VITRO-CARD-1001 study in a population of patients with previous unsuccessful treatment of their wounds and with a median wound duration of 10 months at baseline, a clinically meaningful overall complete healing rate of 30.2% was achieved in the Full Analysis Set (FAS) and a healing rate of 35.6% in the Completer Analysis Set (CAS) by the end of the 12-week treatment period.
A clinically meaningful median percentage wound area reduction of 61.9% between baseline and final assessment was achieved in the FAS and a median of 70.8% in the CAS. At end of study, 31/53 patients (58.5%) had achieved partial healing by at least 50%, 22/53 patients (41.5%) had achieved partial healing by at least 75% and 16/53 patients (30.2%) had complete healing (i.e. 100% epithelialisation).
In the VITRO-CARD-1001 study in addition, to the achievement of clinically meaningful healing and wound area reduction, patients also achieved substantial pain reduction.
Ulcer pain is a common feature of VLUs. A study by Phillips et al. in 1994 found that 65% of patients with VLUs complained of severe pain [11]. In addition, a study by Antignani et al. found that 68% of patients stated that their ulcer created a negative emotional and psychological impact including feelings of fear, social isolation, anger, depression, anxiety, and negative self-image[12].
In the VITROCARD- 1001 study there were 28/53 patients (52.8%) who reported pain at their initial assessment. Of these 20/28 (71.4%) reported no pain at their last assessment (12 weeks, healing or withdrawal, whichever came first). Moreover, 27 of these 28 patients (96.4%) had meaningful pain reduction (i.e. at least 33% reduction in pain) and 22/28 patients (78.6%) had reduction to no pain during the study.
Case study from clinical trial
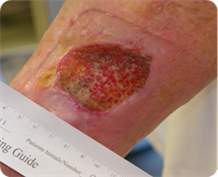
- Ulcer present for 5 years before study entry.
- Ulcer Size = 4.4cm2
- Age = 58 years old
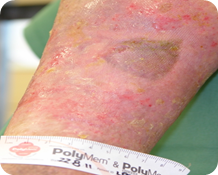
- Re-epithelialised ulcer after 7 weeks of treatment with VitroGro® ECM and standard care.
Long term wound market transformation
Many countries are currently experiencing health budget austerity. Wound care is a growing health issue and there is now unprecedented focus on wound care products to address the clinical and economic burden. We are developing wound care products designed to improve the quality of patient lives and reduce the cost of wound care.
Growth strategy
We see opportunities to have significant impact in wound care markets in Asia, Australia, Canada, Europe, New Zealand, the United States and other markets experiencing the economic burden of wound care. We are also continuing to work with our research and development partners to create more effective, complimentary products for wound care and tissue repair.
Operating model
Tissue Therapies Limited operates with a partnership structure to optimise revenue, flexibility, control and minimise risk. We have partnered with established companies with proven track records.
- Quintiles (European sales force)
- Movianto B.V. (Logistics Hub)
- Catalent S.A. (Manufacturing)
- Eurogentec S.A. (Manufacturing)
- Lime Associates (Supply chain management)
- Queensland University of Technology (Research and Development)
Management systems for design and manufacture
Tissue Therapies Limited is committed to understanding the needs of our customers and patients. We are institutionalizing a culture of quality and performance management into our development and manufacturing control processes. Our management systems are certified in compliance with the requirements of ISO 13485: 2003.
References
[1]Vowden P. Hard to heal wounds made easy. Wounds International 2011
[2] Srinivasaiah N. et al. A point prevalence survey of wounds in the North East of England. J. Wound Care 2007
[3] Vowden KR. and Vowden P. The prevalence, management, equipment provision and outcomes for patients with pressure ulceration identified in a wound care survey with one English health care district. J. Tissue Viability 2009.
[4] Harding K and Queen D. Chronic wounds and their management and prevention is a significant public health issue. Int. Wound J. 2010
[5] Purwins S et al. Cost of illness of chronic leg ulcers in Germany Int. Wound J. 2010
[6] Drew P et al. The cost of wound care for a local population in England. Int. Wound J. 2007
[7] Schultz GS. Extracellular Matrix: review of its roles in acute and chronic wounds. World Wide Wounds. 2005
[8] Widgerow AD . Deconstructing the stalled wound. Wounds 2012
[9] Moor AN. et al. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Rep Reg. 2009
[10] International consensus, Acellular matrices for treatment of wounds. Wounds Int. 2010
[11]Phillips, T. et al. Study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. Journal of the American Academy of Dermatology. 1994
[12]Antignani PL. Classification of chronic venous insufficiency: a review. Angiology. 2001